Malaria

Arteemaxx 100mg Tablet (Artesunate)
Packaging : 4 Tablets in strip ...
Manufactured By : Kachhela M ...
Our Best Price : $1.38 /Piece
Arteemaxx 200mg Tablet (Artesunate)
Packaging : 4 Tablets in strip ...
Manufactured By : Kachhela M ...
Our Best Price : $1.66 /Piece
Arteemaxx 50mg Tablet (Artesunate)
Packaging : 4 Tablets in strip ...
Manufactured By : Kachhela M ...
Our Best Price : $1.04 /Piece
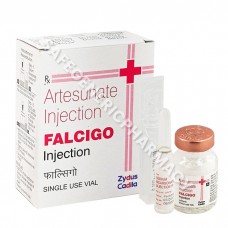
Falcigo 120mg Injection (Artesunate)
Packaging : 1ml in 1 vial
Manufactured By : Zydus Life ...
Our Best Price : $13.50 /Piece
Falcigo 60mg Injection (Artesunate)
Packaging : 1ml in 1 vial
Manufactured By : Zydus Life ...
Our Best Price : $10.00 /Piece
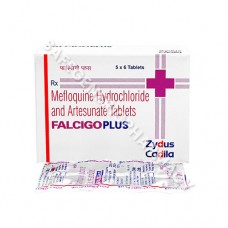
Falcigo Plus Tablet (Artesunate/Mefloquine)
Packaging : 6 tablets in 1 strip
Manufactured By : Zydus Life ...
Our Best Price : $2.43 /Piece
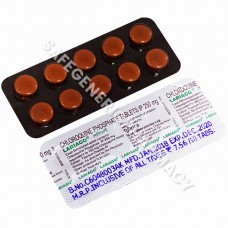
Lariago 250mg Tablet (Chloroquine)
Packaging : 10 Tablets in Strip
Manufactured By : Ipca Labor ...
Our Best Price : $0.44 /Piece
Lariago-DS Tablet (Chloroquine)
Packaging : 5 Tablets in Strip
Manufactured By : Ipca Labor ...
Our Best Price : $0.71 /Piece
Larinate 100 Kit (Artesunate/Sulfadoxine/Pyrimethamine)
Packaging : 5 Tablets in Strip
Manufactured By : Ipca Labor ...
Our Best Price : $4.58 /Piece
Description
Malaria